|
ACETONE
|
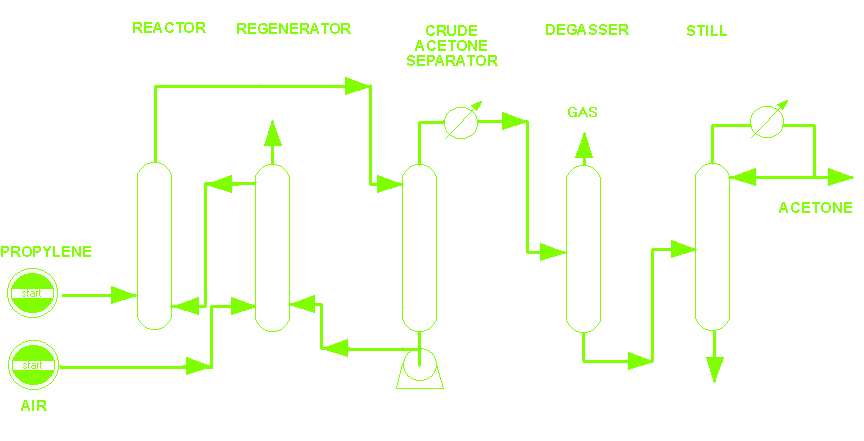
Schematic Acetone Plant.
COSTS
CAPACITY INVESTMENT 2600TMA US$ 3,5MM
LOCALIZACION PRICIO(FOB) EUROPA US$ TM USA US$ TM ASIA-PACIFICO US$ TM
PROCESS. A process for acetone production by direct oxidation of propylene using air.
In this process the catalysis consists of a solution of copper chloride containing small quantities of paladium chloride.
The over all reaction is as follows :
2CH3CH=CH2 + O2 =====> 2CH3COCH3
STEP
In the Reactor
The propyleno and catalyst solution are forced throught a continuous reactor, where practically all propylene is converted in a single stage. The reaction takes place under a moderate pressure and at 100°C. It is exothermic by 61kcal/mole of acetone produced.
The palladium chloride is reduced to elemental palladium and HCl, and is re-oxidized by cupric chloride.
Cupper chloride Water CH3CH=CH2 + 2CuCl2 + H2O =====> CH3COCH3 + 2HCl + 2CuCl
In the Regenerator
The catalyst solution is trated with air by air and recycled. The air used for oxidation depleted almost complety of oxygen, so that the off-air can be used as inert gas.
4CuCl + 4HCl + O2 =====> 4CuCl2 + 2H2O
In the Separator
After pressure reduction, acetone is stripped of catalyst solution.
In the Degasser
In the distillation tower, Crude acetone is separated of propylene and other gas could be produced during the reaction.
In the Still
In the distillation tower, acetone is seperated of any catalyst solution that it could have.
BACK